UK MEDICA is one of the UK pharma distributor companies, registered with the Medical and Healthcare Products Regulatory Agency (MHRA). We are committed to the principles of Good Manufacturing Practice (GMP).

UK MEDICA official patented trade mark in UK
We are expert in the healthcare supplying and distributing patented pharmaceutical products with trade mark of United Kingdom intellectual property office to our pharma distributors (actually “our partners”) all over the world.
Pharma manufacturing Service
UK MEDICA contacts with the most leading pharma manufacturing companies in Europe (ex. COSMOSOL s.r.l.) which not just in the pharma market but even in research, design, production and implementation of new concept of leading-edge Aerosol healthcare and medical device products, which is able to provide innovative packing solutions from both a technical and aesthetic point of view.
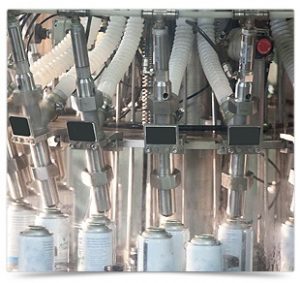
Our decision of “Tailor made” pharmaceutical sprays turns out to be an ever-growing trend, as it meets the need to minimize costs, which is greatly appreciated by the market.
For this reason, we have been structured and perfectly equipped in order to provide our partner distrubutors with the most accurate full-service,in order to meet specific customer´s needs and instructions. That includes:
- Handling of packed components
- Product formulation
- Brands design
- Several quality control procedures as provided for the product requirements and regulations
UK MEDICA Values:
To have got our partners’ job done in a very smoothy way, with accruing of the high appreciation from their end users.
UK Medica manufacturer guarantees the following to our pharma partner distributors :
- Formulation carrying out which labeled on the products.
- European and international legislative assistance.

- Product sensory test.
- Efficiency evaluation and stability test.
- Product/container compatibility test.
- Pilot production.
- Container and/or sticky labels print proofing.
- Packing containers
- Raw materials quality control.
- Bulk production.
- Bulk quality control.
- In-process controls.
- Microbiological controls.
